What Is The Function Of A Catalyst Quizlet . study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. 1.) a catalyst decreases activation energy for a chemical reaction. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. main functions of a catalyst. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. As shown, the catalyzed pathway involves a. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. 2.)catalyst increase the rate of a. the catalyst provides a different reaction path with a lower activation energy.
from www.dreamstime.com
study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. the catalyst provides a different reaction path with a lower activation energy. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. main functions of a catalyst. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. 1.) a catalyst decreases activation energy for a chemical reaction. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. 2.)catalyst increase the rate of a. As shown, the catalyzed pathway involves a.
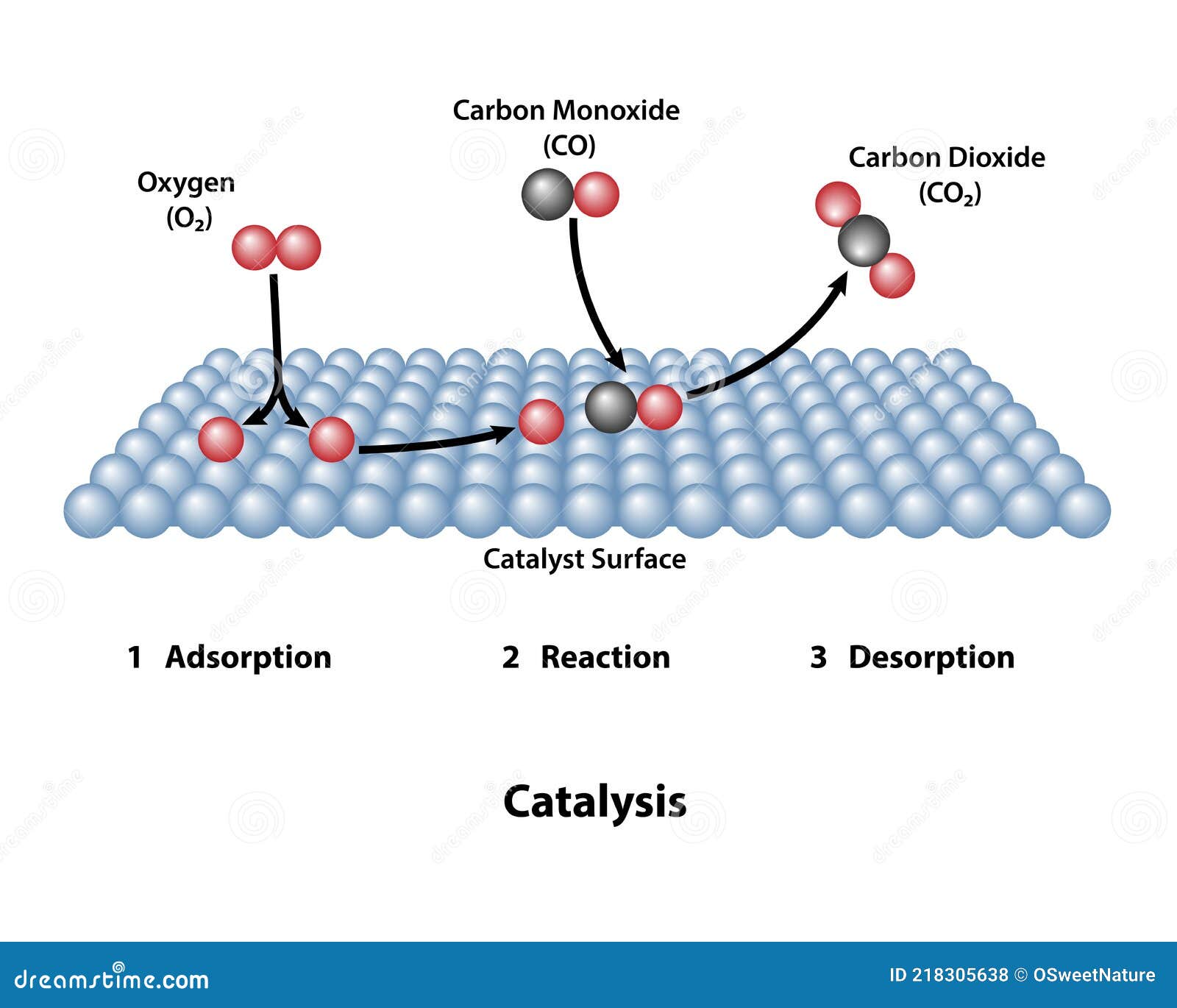
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of
What Is The Function Of A Catalyst Quizlet catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. the catalyst provides a different reaction path with a lower activation energy. 1.) a catalyst decreases activation energy for a chemical reaction. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. main functions of a catalyst. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. 2.)catalyst increase the rate of a. As shown, the catalyzed pathway involves a.
From www.researchgate.net
Schematic structure of catalysts for CMC. (a) hexaaluminate (LaFeAl 11 What Is The Function Of A Catalyst Quizlet As shown, the catalyzed pathway involves a. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. main functions of a catalyst. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. the catalyst. What Is The Function Of A Catalyst Quizlet.
From www.chegg.com
Solved The Diagram Shown Above Shows The Reaction Profile... What Is The Function Of A Catalyst Quizlet main functions of a catalyst. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. the catalyst provides a different reaction path with a. What Is The Function Of A Catalyst Quizlet.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog What Is The Function Of A Catalyst Quizlet the catalyst provides a different reaction path with a lower activation energy. 1.) a catalyst decreases activation energy for a chemical reaction. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. As shown, the catalyzed pathway involves a. 2.)catalyst increase the rate of a. catalysts function by. What Is The Function Of A Catalyst Quizlet.
From www.pinterest.com
Catalyst definition A substance that speeds up a chemical reaction by What Is The Function Of A Catalyst Quizlet 1.) a catalyst decreases activation energy for a chemical reaction. the catalyst provides a different reaction path with a lower activation energy. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. catalysts function by allowing the reaction to take place through an alternative. What Is The Function Of A Catalyst Quizlet.
From www.researchgate.net
Classification of catalyst. Download Scientific Diagram What Is The Function Of A Catalyst Quizlet the catalyst provides a different reaction path with a lower activation energy. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower. What Is The Function Of A Catalyst Quizlet.
From quizlet.com
Catalytic Mechanisms Diagram Quizlet What Is The Function Of A Catalyst Quizlet the catalyst provides a different reaction path with a lower activation energy. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. main functions. What Is The Function Of A Catalyst Quizlet.
From bio1151.nicerweb.com
catalytic.html 08_17CatalyticCycle.jpg What Is The Function Of A Catalyst Quizlet main functions of a catalyst. the catalyst provides a different reaction path with a lower activation energy. 1.) a catalyst decreases activation energy for a chemical reaction. As shown, the catalyzed pathway involves a. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. a catalyst is. What Is The Function Of A Catalyst Quizlet.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction What Is The Function Of A Catalyst Quizlet a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. 2.)catalyst increase the rate of a. the catalyst provides a different reaction path with a lower activation energy. the function of a catalyst is it increases the rate of the chemical reaction by providing. What Is The Function Of A Catalyst Quizlet.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Is The Function Of A Catalyst Quizlet main functions of a catalyst. As shown, the catalyzed pathway involves a. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. a catalyst. What Is The Function Of A Catalyst Quizlet.
From quizlet.com
Enzyme catalyst cycle Diagram Quizlet What Is The Function Of A Catalyst Quizlet a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. 2.)catalyst increase the rate of a. 1.) a catalyst decreases activation energy. What Is The Function Of A Catalyst Quizlet.
From socratic.org
Why does a catalyst cause a reaction to speed up? Socratic What Is The Function Of A Catalyst Quizlet a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that. What Is The Function Of A Catalyst Quizlet.
From quizlet.com
exothermic catalyst profile Diagram Quizlet What Is The Function Of A Catalyst Quizlet catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. 1.) a catalyst decreases activation energy for a chemical reaction. main functions of a catalyst.. What Is The Function Of A Catalyst Quizlet.
From quizlet.com
Enzymes Diagram Quizlet What Is The Function Of A Catalyst Quizlet As shown, the catalyzed pathway involves a. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. 2.)catalyst increase the rate of. What Is The Function Of A Catalyst Quizlet.
From quizlet.com
The data represent x = amount of catalyst added Quizlet What Is The Function Of A Catalyst Quizlet 2.)catalyst increase the rate of a. study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. a catalyst is a chemical substance that affects the. What Is The Function Of A Catalyst Quizlet.
From quizlet.com
Diagram of Enzyme catalyst Quizlet What Is The Function Of A Catalyst Quizlet study with quizlet and memorize flashcards containing terms like catalyst, providing an easier route that has a lower activation. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. 2.)catalyst increase the rate of a. the catalyst provides a different reaction path with a. What Is The Function Of A Catalyst Quizlet.
From jimenunnallyxo.blob.core.windows.net
What Are Catalytic Converters And How Do They Work What Is The Function Of A Catalyst Quizlet the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. As shown, the catalyzed pathway involves a. 1.) a catalyst decreases activation energy for a chemical reaction. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. What Is The Function Of A Catalyst Quizlet.
From www.e-education.psu.edu
Catalytic Reformer FSC 432 Petroleum Refining What Is The Function Of A Catalyst Quizlet catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. main functions of a catalyst. 2.)catalyst increase the rate of a. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. the catalyst provides a different reaction path. What Is The Function Of A Catalyst Quizlet.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of What Is The Function Of A Catalyst Quizlet the function of a catalyst is it increases the rate of the chemical reaction by providing a different pathway with a lower activation. main functions of a catalyst. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. study with quizlet and memorize flashcards containing terms like. What Is The Function Of A Catalyst Quizlet.